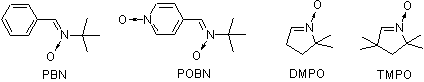
Fig.1
- Spin Traps (General information)
- Nitronylnitroxides as traps of nitric oxide
- The 2H-imidazole-1-oxide spin traps
- Sterically hindered hydroxylamines
- Spin pH-Probes And Labels
- Biradical disulfides as thiol-specific reagents
- 1,2-Diazetine-1,2-dioxides as nitric oxide releasing agents
- Contacts
Characteristic
«Spin probes» is a term for a chemical substances capable of reacting with active free radicals to form persistent paramagnetic compounds, called spin adducts (mainly a nitroxides), the later are registered by means of EPR spectroscopy. Spin probes are used for investigation of various processes in which free radicals play a role. EPR spectra of the spin adducts are used to determine the structure of the free radicals trapped and they provide data about kinetics and mechanisms of free-radical reactions in the system.
The Novosibirsk Institute of Organic Chemistry SB RAS has developed the technologies for production of highly purified spin traps. In decades of scientific research in the field of nitrone chemistry we accumulated unique experience and developed original know-how on methods of synthesis of the spin traps and purification from the admixtures, which are responsible for background signals and artifacts formation.
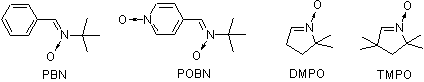
Besides such a widely used spin traps as PBN, POBN, DMPO and TMPO (Fig.1) we produce the original 2H-imidazole 1-oxide spin traps (Fig.2).
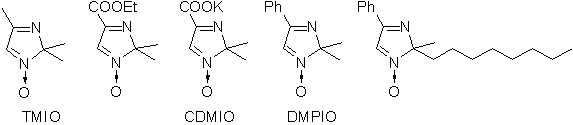
In comparison to other spin traps, these compounds have a number of important advantages: they are of higher chemical stability in various media, they have a high electrochemical oxidation potentials (Ep=1.7-2.5 V vs. s.c.e.) thereby providing more reliable information. Besides that, the spin adducts they form have higher lifetimes and more information can be taken from their EPR spectra.
Technical and Economical Advantages
The spin traps produced in the Novosibirsk Institute of Organic Chemistry SB RAS are inferior to none of the best commercially available samples in quality. We have been preparing the compounds for Acros Organics, Alexis, etc. The world consumption of the spin traps varies from some kilograms (PBN) to some hundreds of grams per year. The demand for the compounds is expected to increase due to development of biomedical research and new applications arising.
Application Area
The spin traps are used for investigation of various free-radical-mediated processes.
Most of the PBN produced is used for beer quality evaluation. Besides that, large amounts of PBN, POBN and DMPO are used in biophysical and biomedical research for investigation of various pathologies development, such as cardiovascular and brain injury during ischemia-reperfusion, cancer, ageing, etc. Intensive studies of protective effects of the nitrone spin traps are carried, implying possible pharmacological applications of these substances.
License Protection
PBN, POBN, DMPO and TMPO are not original products, no patent applications are planned. The patent on the 2H-imidazole 1-oxide spin traps production is no longer supported.
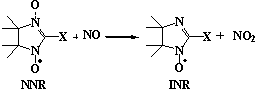
Nitronylnitroxides (NNR) are effective scavengers of nitric oxide in solutions, the rate constants of the reaction are about 104 M-1s-1 [1-3]. The characteristic time of NO trapping by NNR (t<=1 s at [NNR] >= 0.1 mM) is close to the lifetime of NO in vivo. The ability to follow EPR spectra of NNR and INR allows quantitative measurements of concentrations of trapped nitric oxide, the sensitivity of the approach being 1 mM for detection of NO concentration and 0.3 nM/s for the measurements of the rates of NO generation for 1 h in a sample of 0.2 ml. NNR were successfully used to detect nitric oxide release from NO-donors [4-7], from endothelial cells cultures [8], and NO synthesyzed by NO-Synthase in rat cerebellum cytosol [3]. The application of charged NNR incorporated into the inner volume of liposomes seems to be a useful approach to overcome the reduction of NNR in biological samples [3]. Antagonistic action of NNR appears to be helpful both in the studies of pathophysiological role of NO in the treatment of various deseases caused by overproduction of NO [1,9,10]. The NNR and INR can be reduced in vivo or in model systems by superoxide and by other species, but the radicals may be almost quantitatively recovered by oxidation with ferrocyanide or manganese dioxide [11].
- Akaike, T., et al., 1993, Biochemistry, 32, 827-832.
- Joseph, J.; Kalyanaraman, B. and Hyde, J. S., 1993, Bioch. Biophys. Res. Commun., 192, 926-934.
- Woldman, Ya., et al., 1994, Bioch. Biophys. Res. Commun., 202, 195-203.
- Sigh, R.J., et al., 1995, Photochemistry and Photobiology, 61, 325-331.
- Utepbergenov, D. I., et al., 1995, Bioch. Biophys. Res. Commun., 214, 1023-1032.
- Khramtsov, V. V., et al., 1996, Biochemistry (Moscow), 61, 1223-1231.
- Balakirev, M. Yu. and Khramtsov, V. V., 1996, J. Org. Chem. 61, 7263-7269.
- Az-ma, T.; Fujii, K. and Yude, O., 1994, Life Sciences, 54, PL185.
- Konorev, E., et al., 1995, Free Rad. Biol. Med., 18,169.
- Yoshida, M., et al., 1994, Bioch. Biophys. Res. Commun., 202, 293-298.
- Haseloff, R. F., et al. 1997, Fre Rad. Res., 26, 7.
These cyclic aldonitrones have been shown to be useful spin traps, capable of trapping C-, O- and S-centered radicals[1-6]. The conjugated aldonitrone group in these heterocyclic compounds is generally more stable towards both nucleophylic addition onto nitrone carbon atom followed by oxidation, and one-electron oxidation to radical cation (Ep=1.7-2.5 V vs. SCE) followed by nucleophylic addition. This prevents nitroxide artifacts formation, providing more reliable data. The spin traps or their solutions usually can be stored for a reasonable time without significant increase of background signal. Somewhat lower rates of spin trapping for 2H-imidazole-1-oxide spin traps in comparison to DMPO usually does not make problem in measurements because of higher lifetimes of the spin adducts[1,2,4]. Surprisingly, the spin adducts are 2.5 times more stable then those of DMPO even in a nitroxide-reducing ascorbate-containing systems[1,4,6]. Lower reactivity of 2H-imidazole-1-oxide spin traps results in higher selectivity, e.g. the spin traps are completely not reactive towards superoxide addition. They usually form less spin adducts with O-centered radicals and more spin adducts with C-centered radicals than DMPO under similar conditions[4].
EPR parameters of the spin adducts formed by 2H-imidazole-1-oxide spin traps are highly sensitive to trapped radical structure. Due to nearly planarity of 3-imidazoline ring of the resulting spin adducts they have a very sharp lines. This permits observation of additional splitting on a-hydrogen atoms of trapped primary alkyl radicals[6].
Various 2H-imidazole-1-oxide derivatives were successfully used for spin trapping. The 2,2,4-trimethyl-2H-imidazole-1-oxide (TMIO) [1-7] is the most investigated one. It resembles DMPO in physical properties, solubility, lipophilicity and cytotoxicity level. Following derivatives should be also mentioned:
- 4-Carboxy-2,2-dimethyl-2H-imidazole-1-oxide, potassium salt
- This is a highly hydrophylic compound, a salt of strong acid (pK ca. 1), does not permeate membranes. It can not trap radicals generated inside micelles or liposomes.
- 2,2-Dimethyl-4-phenyl-2H-imidazole-1-oxide [1-5]
- This spin trap was shown to be located on the surface of membranes. Moderately soluble in water.
- 2-Methyl-2-octyl-4-phenyl-2H-imidazole-1-oxide [3,5]
- Highly lipophylic compound.
- 2-(2-Carboxyethyl)-2-methyl-4-phenyl-2H-imidazole-1-oxide
- Acid, under neutral pH highly soluble in water (as a salt), having partition coefficient octanol/water about 1. The spin adducts can be concentrated by reversible extraction (from acidified water medium by organic solvent and than back into alkaline solution). Does not produce strong cytotoxic effects [7].
These compounds were suggested for efficient spin trapping of superoxide radical and peroxinitrite [10-13].
- S.I. Dikalov, et al. Bull. Rus. Acad. Sci.. Div. Chem. Sci.. 1992, (41), 834.
- E. Klauschenz, et al. Free Rad. Res. 1994, 20 (2), 103.
- G. Strul, et al. J.C.S. Perkin trans. 2. 1993, (11), 2057.
- S.I. Dikalov, et. al. B. B. R. C. 1996, 218, 616.
- G. Strul, et al. J.C.S. Perkin trans. 2. 1994, (6), 1229.
- R. F. Haseloff, et al. Free Rad. Res. 1997, 26, 159.
- R. F. Haseloff, et al. FEBS Lett. 1997, 418, 73.
- G. I. Skubnevskaya, et al. Izv. Akad. Nauk SSSR, Ser. Khim. 1987, (2), 312.
- G.G. Dultseva, et al. Izv. Sib. Otd. Akad. Nauk SSSR, Ser. Khim. Nauk 1989, (1), 77.
- S.I. Dikalov, et. al. B. B. R. C. 1997, 231, 701
- S.I. Dikalov, et. al. Nitric Oxide: Biol. Chem. 1997, 1 (5), 423.
- S.I. Dikalov, et. al. B. B. R. C. 1998, 248, 211
- B. Fink, et al. Free Rad. Biol. Med., 2000, 28(1), 121.
Back
Information about compounds
Characteristic
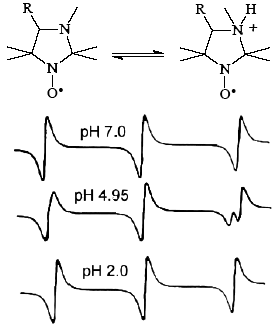
Nitroxides with pH-dependent EPR spectra have been successfully used as pH-sensitive spin probes for determination of local pH and for investigation of proton-related transport processes in various systems including biological objects, heterogeneous nontransparent samples and biophysical models. Imidazoline and imidazolidine nitroxides are the most effective spin pH-probes useful for measurement of local pH-values within the range from 0 to 14, the accuracy of determination is 0.05 pH units [1,2]. They have been applied for pH monitoring in nontransparent “water in oil” systems [3] and liposomes [4], pH-measurements in the interior of polyelectrolites [5] and of zeolites [2], studies of transmembrane transport processes [5,6], surface electrostatics of membranes and proteins[7], in vivo ESR measurement of pH in biodegradable polymers [8]. Low-field EPR techniques permit noninvasive pH-measurements in living organisms, e.g. monitoring of pH changes in stomach of a living rat using pH-sensitive spin probes [9].
- Khramtsov, V. V. and Weiner, L. M., 1988, Proton exchange in stable nitroxyl radicals: pH-sensitive spin probes. In: Imidazoline Nitroxides (ed. Volodarsky, L. B.), CRC Press, Boca Raton, V. 2, 37-80.
- Khramtsov, V. V. and Volodarsky, L. B., 1997, Use of imidazoline nitroxides in studies of chemical reactions: ESR measurements of the concentration and reactivity of protons, thiols and nitric oxide. In: Biological Magnetic Resonance, Vol. 14; Berliner, L. J., Ed.; Plenum Press: New York, 1998, 109.
- Kroll, C., et al., 1995, European J. Pharmaceutical Sciences, 3, 21.
- Balakirev, M.Yu. and Khramtsov, V. V., 1993, J. Chem. Soc. Perkin Trans. 2., 2157-2160.
- Molochnikov, L. S.; Kovalyova E. G.; Grigor’ev, I. A.; Reznikov V. A. Determination of acidity in in the interior of the cross-linked polyelectrolyte grain by the use of pH-sensitive probes. In: Metal-containing polymeric materials. Ed. C. U. Pittman, Jr. et al. Plenum Press, NY. 1996, P. 395-401.
- Khramtsov, V. V.; Panteleyev, M. V. and Weiner, L.M., 1989, J. Bioch. Biophys. Methods, 18, 237-246.
- Khramtsov, V. V., et al., 1992, Bioch. Biophys. Acta, 1104, ,317-323.
- Màåder, K., et al., 1996, Biomaterials, 17, 457-461.
- Khramtsov, V. V.; Grigor’ev, I. A.; Foster, M. A.; Lurie, D. J.; Nicholson, I. Cell. Mol. Biol., 2000, 46, 1361.
- I. A. Kirilyuk, T. G. Shevelev, D. A. Morozov, E. L. Khromovskih, N. G. Skuridin, V. V. Khramtsov and I. A. Grigor'ev, Synthesis, 2003, (6), 871-878.
- I. A. Kirilyuk, A. A. Bobko, I. A. Grigor'ev and V. V. Khramtsov Org. Biomol. Chem. 2004, 2, 1025-1030.
- I. A. Kirilyuk, A. A. Bobko, V. V. Khramtsov and I. A. Grigor'ev Org. Biomol. Chem. 2005, 3, 1269-1274.
Back
Information about compounds
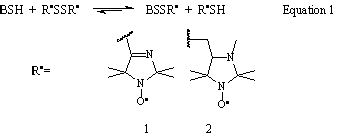
The disulfide biradicals with imidazoline (1) or imidazolidine (2) nitroxide fragments have been used as thiol-specific reagents for EPR determination of SH-groups in high- and low- molecular weight compounds according to eg. 1 [1,3]. The method allows determination of thiols in 0.1 - 1 mM concentration even in colored and highly absorbing samples and measurement of the rate constants of the reaction of the biradical with the thiol. The method proved to be highly sensitive and reproducible for intracellular glutathione measurement [1,4,5], characterisation of thiols damage during oxidative stress [6], measurement of the rate constants of the reaction of thiols with superoxide radical [7], and for thiol measurement in isolated organs [8]. the method was applied to measure the rate of ensymatic reactions, the products or substrates of which are thiols [9], and for reversible modification of proteins [1-3]. The imidazolidine biradical (2) can be applied in low concentrations at physiological conditions, which seems to be of particular interest for noninvasive thiols measurement both in vitro and in vivo [2].
- Khramtsov, V. V. et al., 1989, Analyt. Biochem., 182, 58-63.
- Khramtsov, V. V. and Volodarsky, L. B., 1997, Use of imidazoline nitroxides in studies of chemical reactions: ESR measurements of the concentration and reactivity of protons, thiols and nitric oxide. In: Spin Labeling. Next milleunium. (Ed. L. J. Berliner) Plenum Press, N.Y., Ch. 4.
- Weiner, L. M., 1995, Metods Ensymol. 251, 87-105.
- Weiner, L. M.; Hu, H.; Swartz, H. M., 1991, FEBS Lett., 290, 243.
- Busse, E.; Zimmer, G. and Kornuber, B., 1992, Strahlentherapie und Onkologie, 168, 419-423.
- Busse, E.; Zimmer, G. and Kornuber, B., 1993, Arzheim.-Forsh./Drug Res., 43, 378-381.
- Yelinova, V. I., et al., 1996, Bioch. Biophys. Res. Commun., 221, 300-303.
- Nohl, H.; Stolze, K. and Weiner, L. M., 1995, Metods Ensymol., 251, 191.
- Khramtsov, V.; Gorjunova, T. and Weiner, L. M., 1991, Bioch. Biophys. Res. Commun., 179, 520-527.
Back
Information about compounds
3,4-Dihydro-1,2-diazete 1,2-dioxides (diazetine dioxides, DD) are known to liberate nitric oxide during spontaneous decomposition [1-3]. 3-Bromo-DD were also shown to react with thiols with nitric oxide formation [4]. Strong vasorelaxant and antiaggregant activities of DD derivatives were described, 3-bromo-DD derivatives being the most active [1-6]. Recent data support the conclusion that high NO-mediated activity of 3-bromo-DD is caused by thiol-induced NO release from these derivatives in the presence of endogenous thiols [4]. Cytotoxic effects of DD were observed at 2 orders of magnitude higher concentrations than those sufficient for significant vasorelaxation [4].
- Severina, I. S., et al., 1993, Biochem. Mol. Biol. Int., 30, 357-366.
- Utepbergenov, D.I., et al., 1995, Bioch. Biophys. Res. Commun., 214, 1023-1032.
- Ryaposova, I. K.; Grigoryev, N. B. and Severina, I. S., 1994, Biokhimiya (Moscow), 59, 537-542.
- Kirilyuk, I. A., et al., 1997, J. Med. Chem. (in press).
- Severina, I. S.; Belushkina, N. N. and Grigoryev, N. B., 1994, Biochem. Mol. Biol. Int., 33, 957-967.
- Belushkina, N. N.; Grigoryev, N. B. and Severina, I. S., 1994, Biokhimiya (Moscow), 59, 1689-1697.
Back
Information about compounds
| Family name | Grigor’ev |
| First name | Igor Alexeyevich |
| Position, academic degree | Director, Prof. |
| Organization | N. N. Vorozhtsov Novosibirsk Institute of Organic Chemistry SB RAS |
| Address | Academician Lavrentiev Ave., 9 |
| City | Novosibirsk |
| Postcode | 630090 |
| Phone | +7-(383)-3308852 or -3307387 |
| Fax | +7-(383)-3309752 |
| grig@nioch.nsc.ru or benzol@nioch.nsc.ru |